Crystalline Solids: Think of crystalline solids like neatly arranged LEGO blocks. In these solids, the particles (atoms, molecules) are organized in a regular and repeating pattern, just like the specific way you stack LEGO blocks together. It’s like having a well-ordered structure.
Amorphous Solids: Now, imagine a messy pile of marbles. Amorphous solids are a bit like that. The particles are still stuck together, but they don’t have a specific order. It’s like a jumble, not following a pattern. So, amorphous solids lack the tidy arrangement that crystalline solids have.
Crystalline Solids
Crystalline solids exhibit a highly ordered arrangement of atoms or molecules in a repeating three-dimensional pattern known as a crystal lattice. This ordered structure gives rise to several key characteristics:
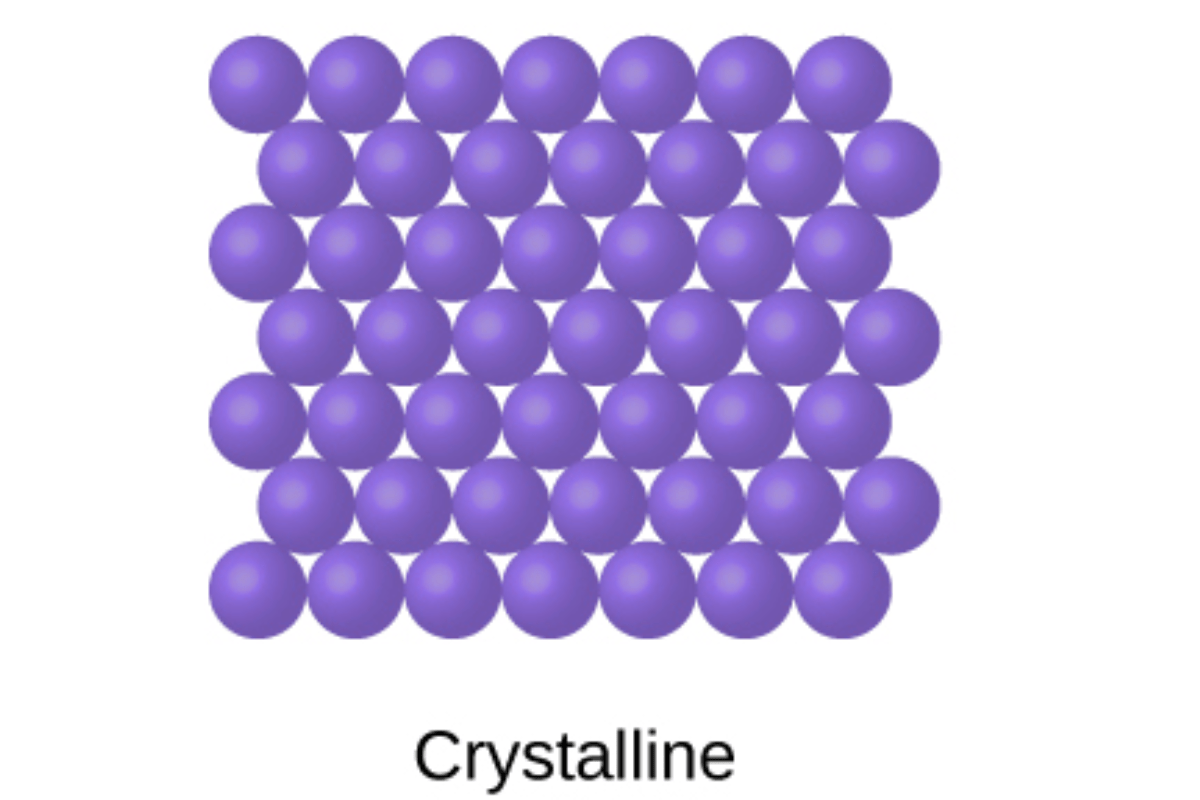
Ordered Structure: At the heart of crystalline solids lies a regular and repeating pattern of atoms or molecules. This long-range order extends throughout the entire material, imparting a high degree of structural integrity and uniformity. Each crystal lattice possesses distinct symmetry elements that dictate its overall shape and properties.
Distinct Melting Point: One defining feature of crystalline solids is their well-defined melting point. As temperature increases, the thermal energy overcomes the interatomic forces holding the lattice together, leading to a phase transition from solid to liquid. This transition occurs at a specific temperature characteristic of the material’s crystal structure.
Anisotropic Properties: Crystalline solids often exhibit anisotropic properties, meaning their physical and chemical characteristics vary with crystallographic direction. This anisotropy arises from the preferential alignment of atoms or molecules along specific crystal planes. As a result, mechanical, electrical, and optical properties may differ significantly depending on the direction of measurement.
Examples: Common examples of crystalline solids include metals, salts, and semiconductor materials such as silicon (Si) and gallium arsenide (GaAs). These materials serve as the foundation for various semiconductor devices, ranging from transistors to solar cells, owing to their well-defined structures and predictable behaviors.
Amorphous Solids
In contrast to crystalline solids, amorphous solids lack long-range order in their atomic or molecular arrangement. Instead, they exhibit a more random and disordered structure characterized by the following features:
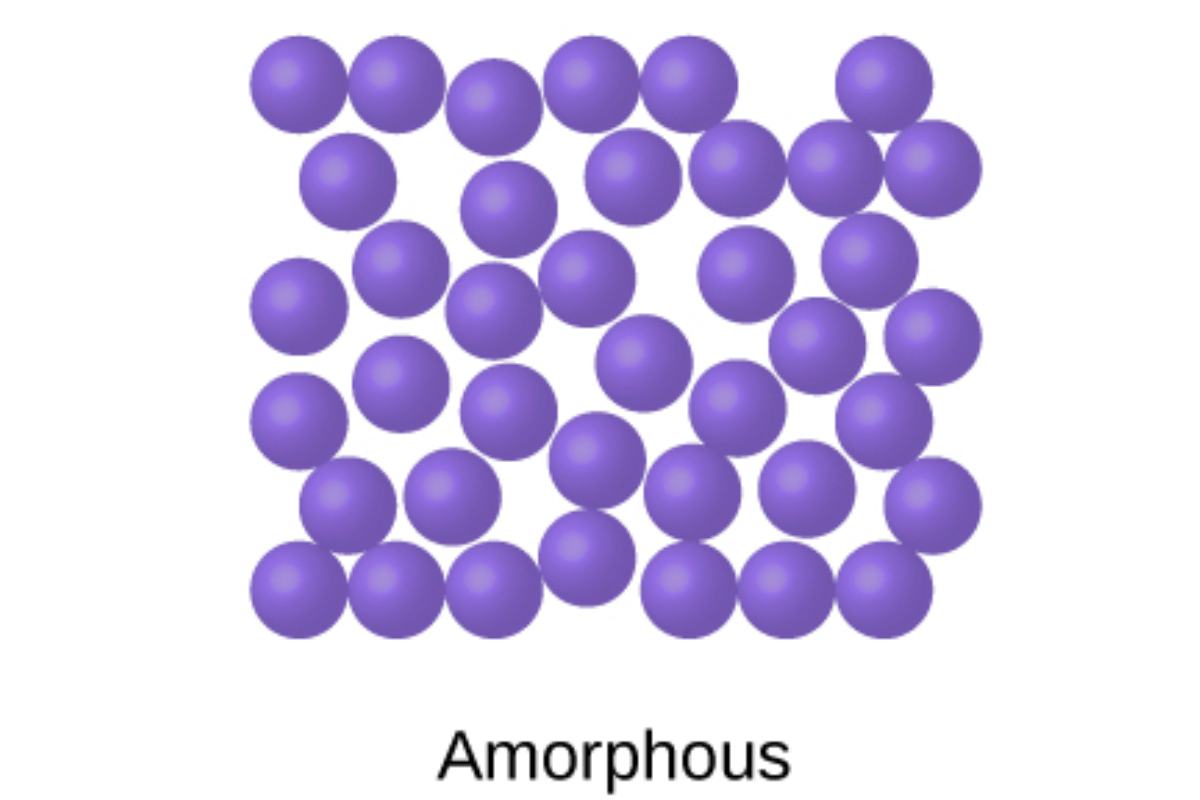
Random Structure: Amorphous solids lack the periodicity and regularity inherent in crystalline lattices. Instead, their atomic or molecular arrangement is akin to a jumbled puzzle, with no discernible pattern extending over long distances. Despite this disorder, short-range order may still exist within limited spatial regions.
No Distinct Melting Point: Unlike crystalline solids, amorphous solids do not exhibit a sharp melting point. Instead, they undergo a gradual softening process over a range of temperatures. This behavior stems from the absence of well-defined crystal planes and the presence of structural defects, which hinder the orderly transition from solid to liquid.
Isotropic Properties: Amorphous solids typically display isotropic properties, meaning their physical and chemical attributes remain uniform in all directions. This isotropy arises from the random arrangement of atoms or molecules, which eliminates directional dependencies observed in crystalline materials.
Examples: Amorphous solids encompass a diverse range of materials, including glasses, certain polymers, and semiconductor substances such as hydrogenated amorphous silicon (a-Si:H). These materials find applications in thin-film transistors, optical coatings, and photovoltaic devices, capitalizing on their ease of fabrication and versatile properties.
Differences between Crystalline Solids and Amorphous Solids
| Characteristic | Crystalline Solids | Amorphous Solids |
|---|---|---|
| Particle Arrangement | Particles are arranged in a regular, repeating pattern. | Particles lack a specific order and are randomly arranged. |
| Melting Point | Have a distinct and sharp melting point. | Tend to soften over a range of temperatures without a sharp melting point. |
| Transparency | Can be transparent or translucent. | Can be transparent or opaque. |
| Mechanical Properties | Tend to have a well-defined structure, resulting in distinct mechanical properties. | Lack a specific structure, leading to less defined mechanical properties. |
| Examples | Examples include salt, diamond, and quartz. | Examples include glass, rubber, and some plastics. |
| Heat Conduction | Generally, good conductors of heat. | Variable heat conduction properties. |
| Breakage Pattern | Tend to break along distinct planes or surfaces. | Break more randomly without well-defined planes. |
| Reproducibility | Have a repeating and reproducible pattern. | Lack a repeating pattern, making reproduction challenging. |
| Crack Propagation | Crack propagation tends to be more predictable. | Crack propagation can be less predictable. |
| Examples of Natural Forms | Crystals in snowflakes and minerals are crystalline. | Glass and certain gels are examples of amorphous forms. |